What Is the Carbon(C) Electron Configuration?
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Electron Configuration Electron Shell Valence Electron Carbon, PNG
10: Electrons in Atoms 10.6: Valence Electrons

5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence
Each Oxygen atom has 6 valence electrons whereas the Carbon atom only has 4 valence electrons. To satisfy the Octet Rule, Carbon needs 4 more valence electrons. Since each Oxygen atom has 3 lone pairs of electrons, they can each share 1 pair of electrons with Carbon; as a result, filling Carbon's outer valence shell (Satisfying the Octet Rule).

Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
Description The valence is the combining capacity of an atom of a given element, determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1.
How to Find the Valence Electrons for Carbon(C)?
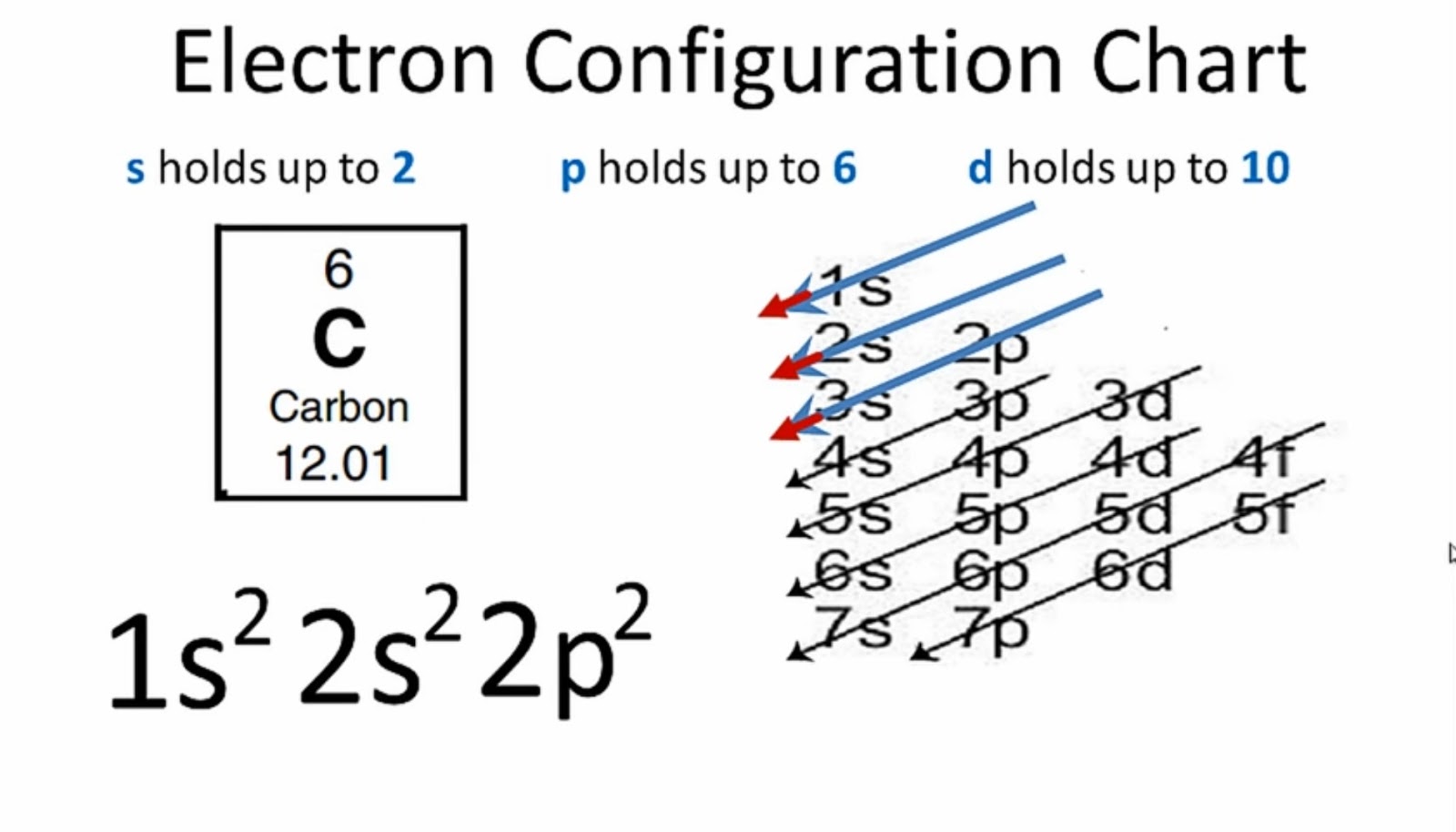
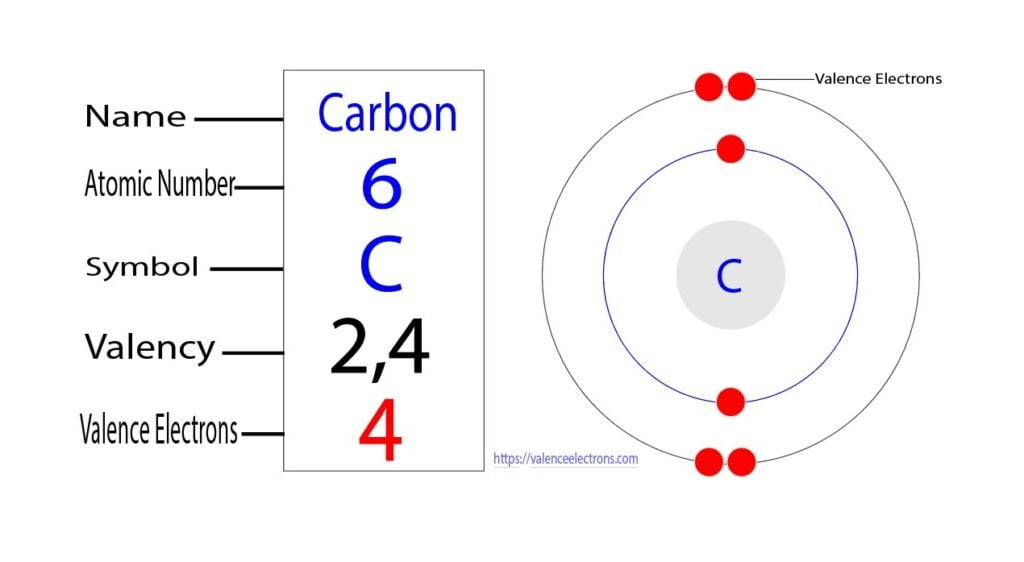
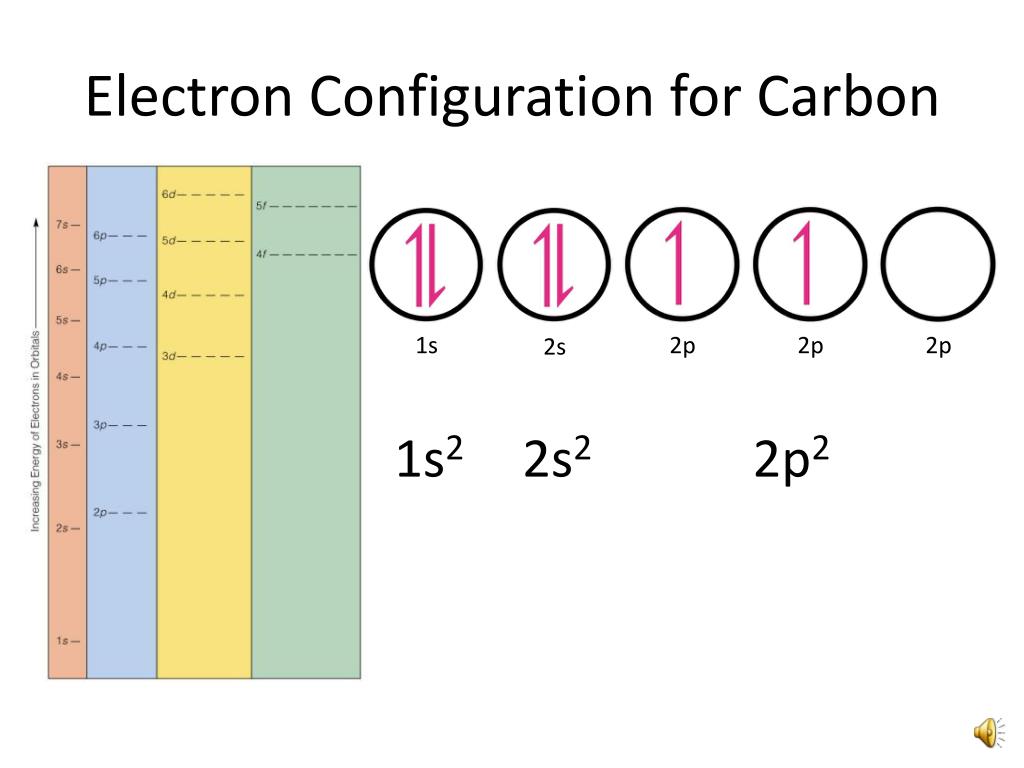
Method 1: According the electron configuration of carbon atom, 1s 2 2s 2 2p 2 There are 4 valence electrons (2s 2 2p 2) in the outermost shell of the carbon atom. Method 2: The valence electron of the carbon can be determined using a Periodic Table. Each column in the Periodic Table represents a group of elements.
Carbon Has Four Valence Electrons And The Lewis Structures Of Methane
The periodic table By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns— groups —and rows— periods —that share certain properties.

High diagram shows the valence electron pattern for a carbon atom
The valence electron configuration of carbon atom is 2s 2 2p 2 as shown in the orbital diagram. Figure 1.6g Orbital diagram of valence electrons in carbon atom. Based on the valence bond theory, with two half-filled orbitals available, the carbon atom should be able to form two bonds. However, carbon always has four bonds in any stable organic.
How to Find the Valence Electrons for Carbon Disulfide?
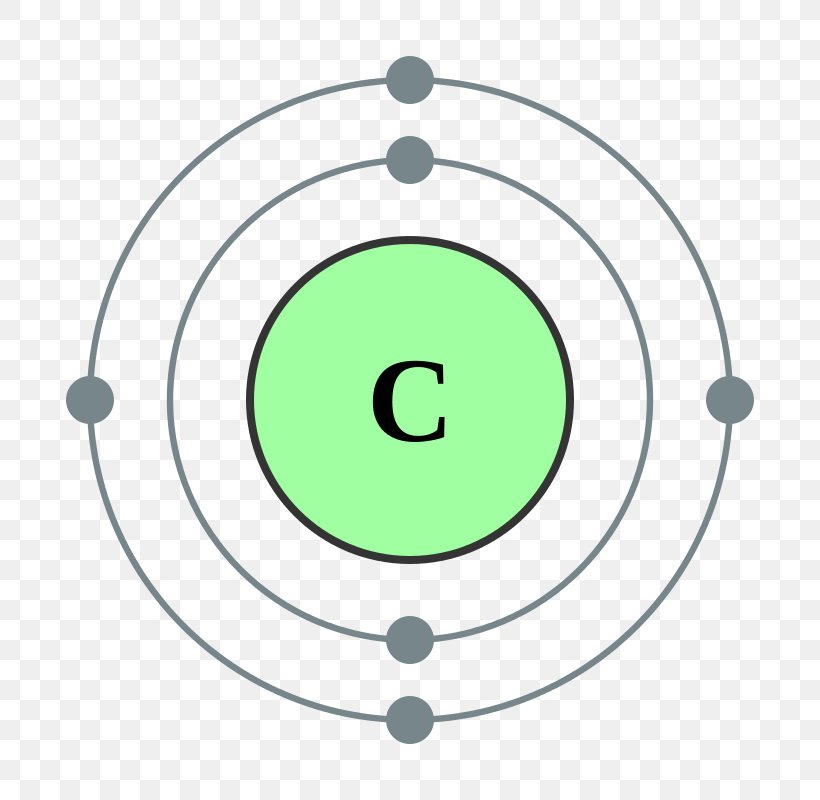
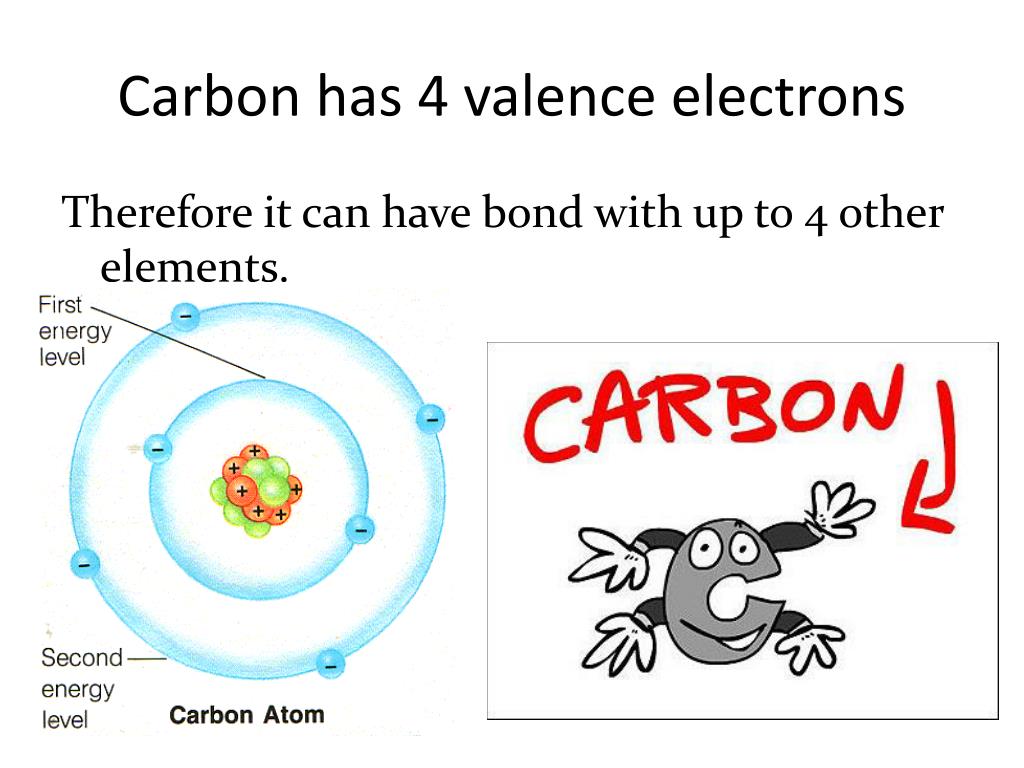
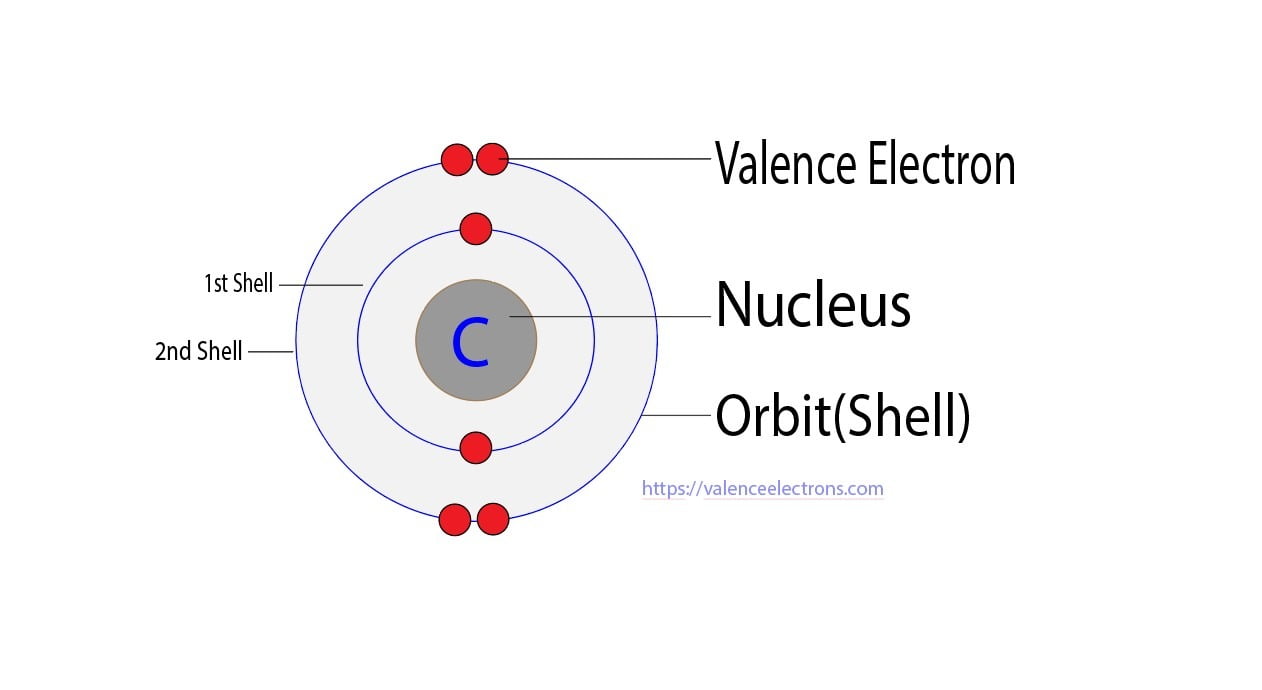
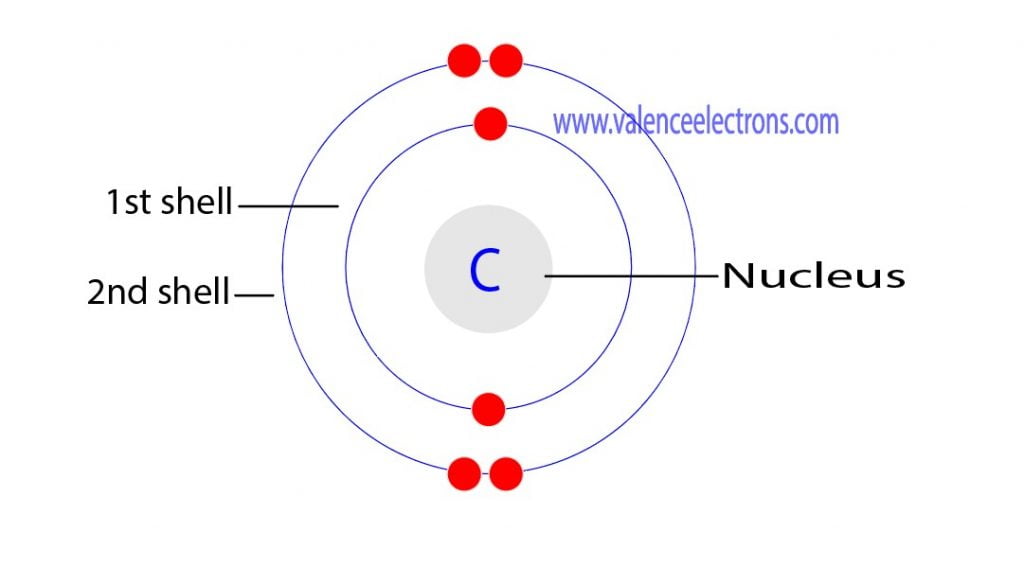
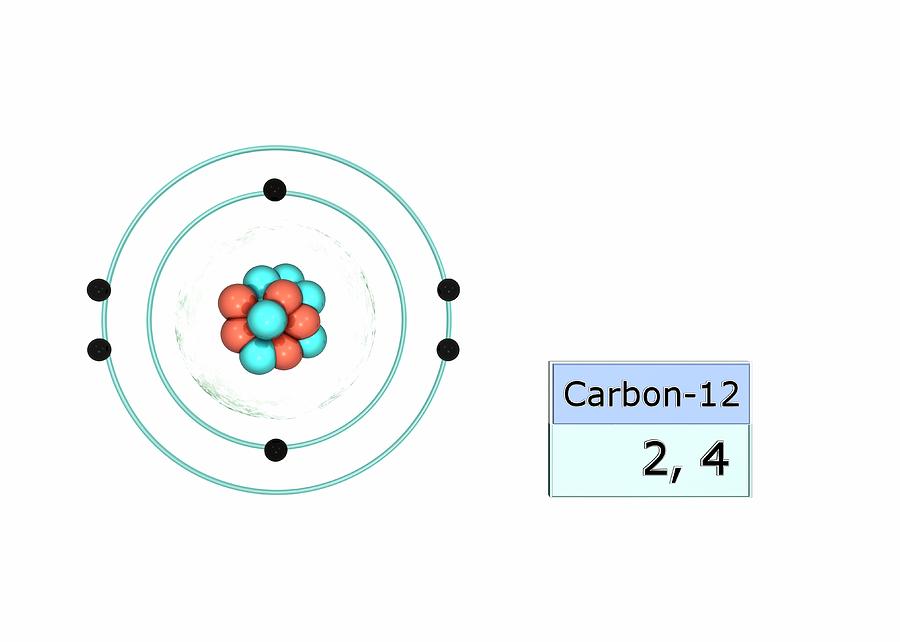
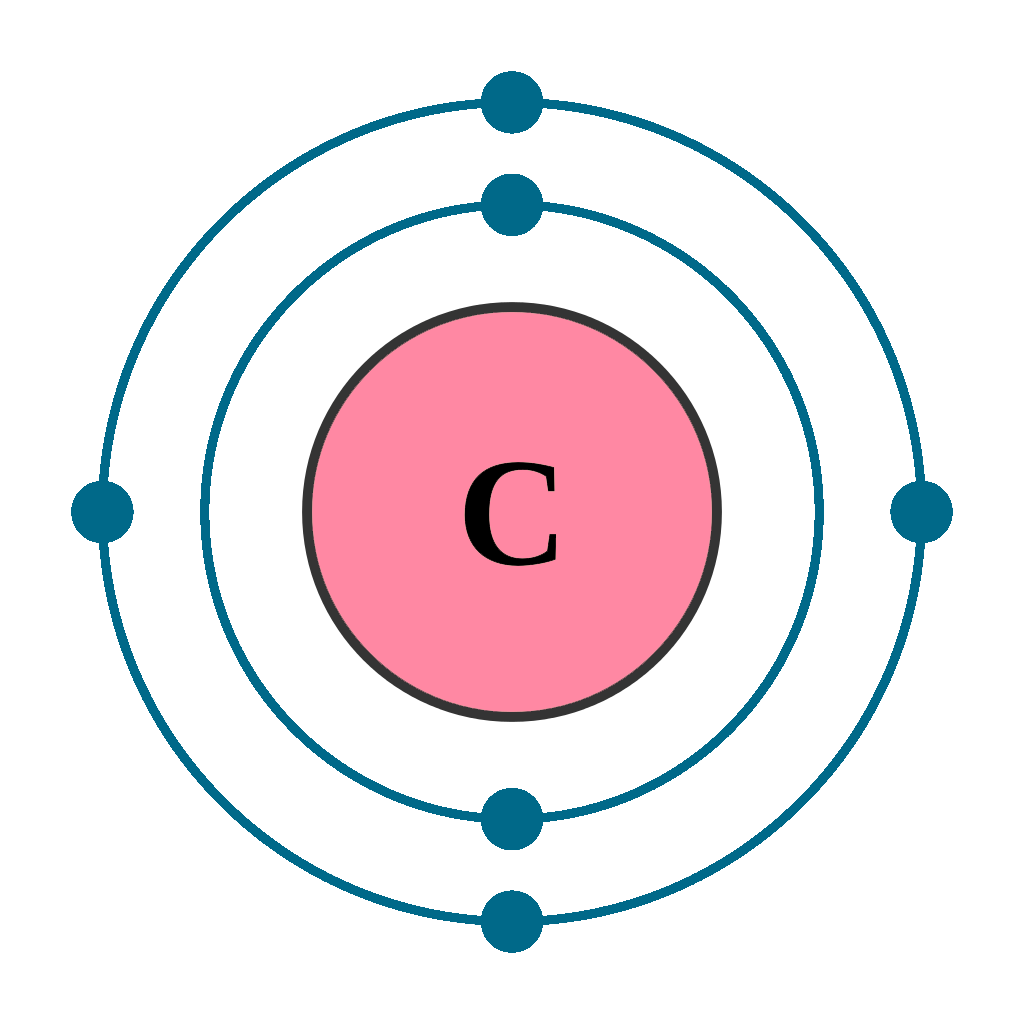
Carbon has an atomic number of six (meaning six protons, and six electrons as well in a neutral atom), so the first two electrons fill the inner shell and the remaining four are left in the second shell, which is the valence (outermost) shell.
Electron Configuration for Carbon (C, C4−) Full Guide
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Carbon Electron Configuration Photograph by Photo
1 Find a periodic table of elements. This is a color-coded table made up of many different squares that lists all of the chemical elements known to humankind. The periodic table reveals lots of information about the elements — we'll use some of this information to determine the number of valence electrons in the atom we're investigating.
Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron.

How many valence electrons does a carbon atom have? YouTube
The electronic configuration of Carbon C can be written as 1 s 2 2 s 2 2 p 2. The valence electrons are the sum of the electrons in the outermost shell, that is two 2 s electrons and two 2 p electrons which gives a total of four valence electrons. Therefore, the valence electron in a Carbon C atom is 4. Suggest Corrections.
PPT Orbital Filling Electron Configurations PowerPoint Presentation
Explanation: To find how many valence electrons are in an atom, you can look at the periodic table: Look at the writing above each Group, or column. The number next to the "A" is the number of valence electrons in an atom of an element in that Group. Carbon is in Group 4A, so it has 4 valence electrons. Answer link

Carbon — Role and Importance to Life Expii
Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond. The Octet Rule The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.

Aufbau Diagram and Electron Configuration for Carbon Cumming Wherieving
Flag Just Keith 9 years ago Helium is not truly a member of Group 18 (or Group VIII in the older system). We just place it there because it is unique and does not really fit into any group. It is placed there because it is practically inert, like the other noble gases. Not all elements follow the octet rule.

Periodic Table With Valence Electron Numbers
1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2. Arrange the atoms to show specific connections.